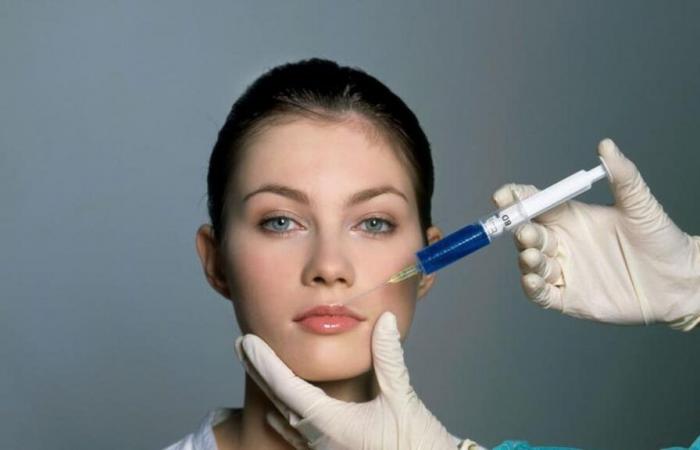
With the increase in landfill implants based on hyaluronic acid, the use of hyaluronidase has increased to correct unsatisfactory results, But not all products with this ingredient are suitable for that purpose: a bad praxis is being made.
“Bad praxis is assuming a serious risk to human Health,” the Spanish Society of Aesthetic Medicine (SIME)who points out that “his choice must be done correctly and in accordance with current regulations.”
Hialuronidase – enzyme that decomposes hyaluronic acid – is present in the market both in the form of cosmetic product and medication, and in both cases it usually occurs in similar formats, such as roads or blisters.
This similarity has given rise to confusion and cases have been detected in which some clinics have injected hyaluronidase marketed as cosmetic, which is a serious risk to public health.
It is essential to differentiate both types of products to avoid confusion about their use and application. The Spanish Agency for Medicines and Health Products (AEMPS) reports on the correct use of hyaluronidase.
Products containing hyaluronidase can only be marketed as cosmetics if they have a cosmetic function and their application is topical.
The administration of this cosmetic product by injection or other intradermal or systemic pathways, which allow an application of the deepest product of the epidermisas is the case in microinjection or mesotherapy techniques, among other treatments, it is not allowed and involves a serious risk to human health.
In order to guarantee a safe use of these cosmetic products that are presented in vials or ampoules, their labeling should include: “topical use, not to inject.”
-Currently, in Spain there is no registered medication with hyaluronidase. However, centers that wish to acquire hyaluronidase can carry out the application process as a foreign medicine through the medication service in special situations of the AEMPS.
The use of hyaluronidase injectable is restricted only to products marketed as medicines, and its administration must be in charge of qualified health professionals.
Given the bad praxis that is being carried out, the citizen must ask the professional with information about the product he will use in his procedure.
The administration of landfill implants based on hyaluronic acid, as well as the use of injectable hyaluronidase, must be performed exclusively by duly qualified doctors and with specific training in these techniques.
When worked with stuffing supplants based on hyaluronic acid, the aesthetic professional must have hyaluronidase as a medicine to be able to act immediately to possible complications.
You must also verify that product labeling, as well as the function of the same and follow the warnings that appear in it.
It must be taken into account that the intraderm or systemic injection or application of a cosmetic product is not allowed. This practice is a serious risk and constitutes a bad praxis, which may be subject to administrative, civil and criminal responsibilities.
In case of doubt about the consideration of the product as cosmetic or medication, the professional must consult the AEMPS, EFE reports.