The reports were presented to ANMAT within the framework of the corresponding regulatory approval process.
These are PanBio, developed by Drofar and Abbott, intended to be self-administered by non-professional users (sold through the Mitest website), and CheckNow, the same test, for use in public health.
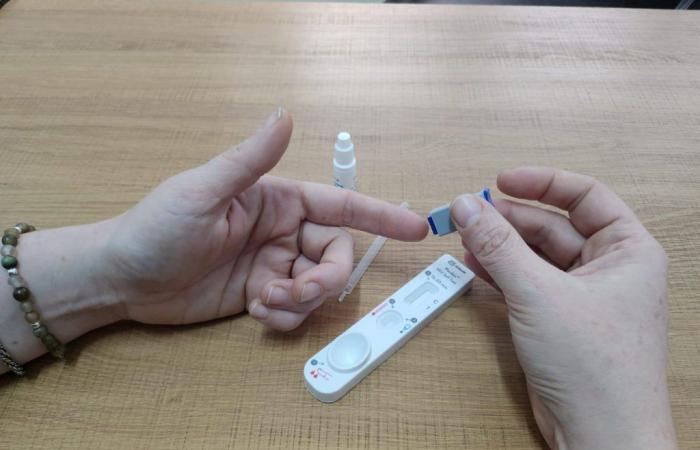
Both kits use a capillary blood sample, obtained from a digital puncture (that is, from the ring, middle or index finger) that only requires three components: the test device, a lancet for the puncture and a buffer solution.
To evaluate these tests, parallel studies were designed with a double objective: on the one hand, measure the sensitivity and specificity of the kits; and on the other, observe its usability in untrained users.
The tests were evaluated by both professional users and non-professional users.
First, a round of tests was carried out with frozen serum samples stored in a non-binding, anonymous manner, previously characterized, both with the kit under study and with the reference method.
70 positive samples were analyzed, which were correctly identified by both reagents (CheckNow and PanBio), and 105 negative samples, among which there was only one false positive result for both tests. This implies a sensitivity of 100% and a specificity of 99.05% for each one.
Subsequently, 40 non-professionals were invited to test the kit, after explaining the purpose of the study, risks and benefits. It should be noted that, in addition to the self-test, the participants were able to access a conventional rapid test test carried out by the Central Laboratory, so that they could leave with a diagnosis confirmed by both methods.
The participants performed the test according to the instructions contained on the device, with a professional from the research team as an observer, who supervised the tests and the interpretation of the results, but did not help the volunteers. When each participant completed their test, the observer confirmed the result obtained.
Users also completed a 30-question questionnaire to evaluate acceptability, fidelity, and usability of the device.
All lay users understood the test procedures, perceived that they used the test correctly, and stated that they would use similar devices at home.
Furthermore, the valid results were correlated with those obtained by the test carried out by professionals. Only one invalid test was obtained in the tests intended for use in public health.
The study concluded that the tests were easy to use, safe and reliable for lay people.
From the company, they highlighted that they chose to request this validation from the Central Laboratory of the province due to their execution capacity and quality of work. It should be noted that the institution is a reference throughout the province and also at the national level in the diagnosis and monitoring of people living with HIV, and has been a pioneer in decentralization and diagnostic expansion strategies, together with the Provincial HIV Program.
The development of this self-test and its approval in Argentina represent an important step to increase access, given that they not only facilitate the acquisition of these tests, but also resolve specific diagnostic demands in a context of maximum discretion.
In addition, they have the potential to increase the acceptance of testing and to empower people who for various reasons do not approach or encounter barriers in the health system, especially in key populations and other people at high risk of HIV infection.